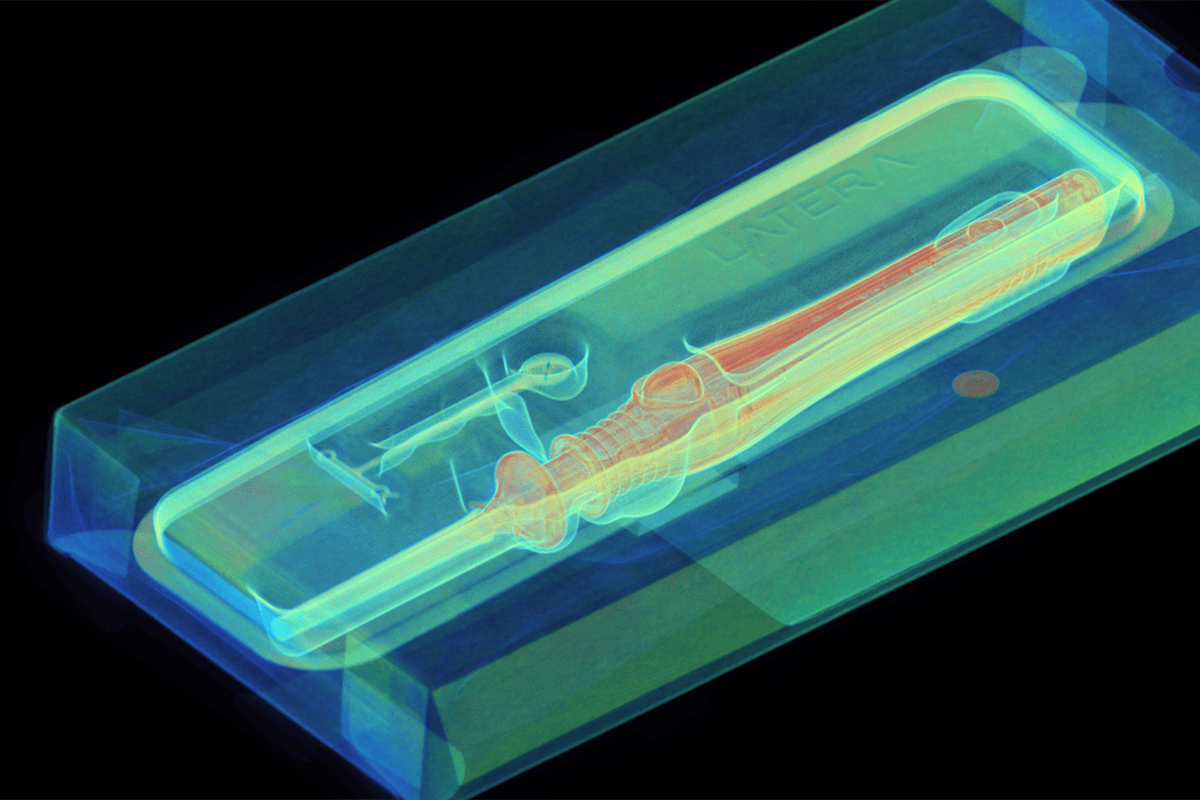
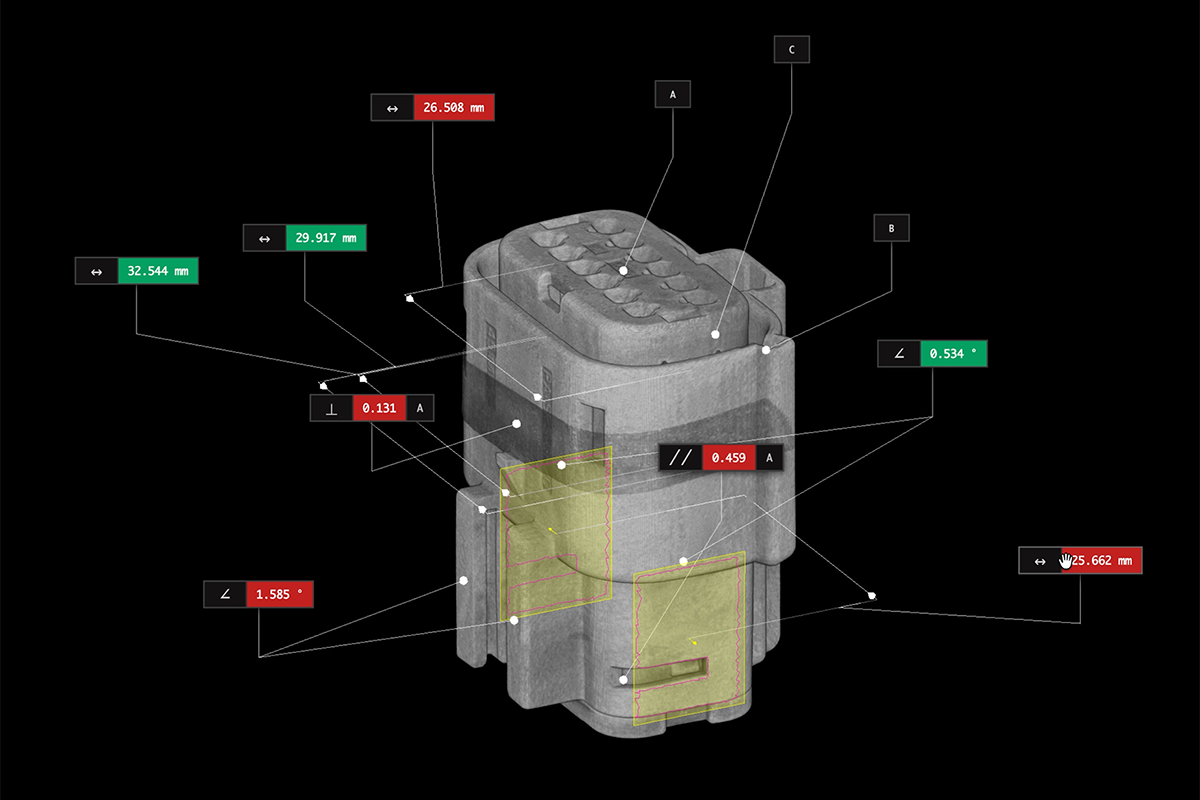
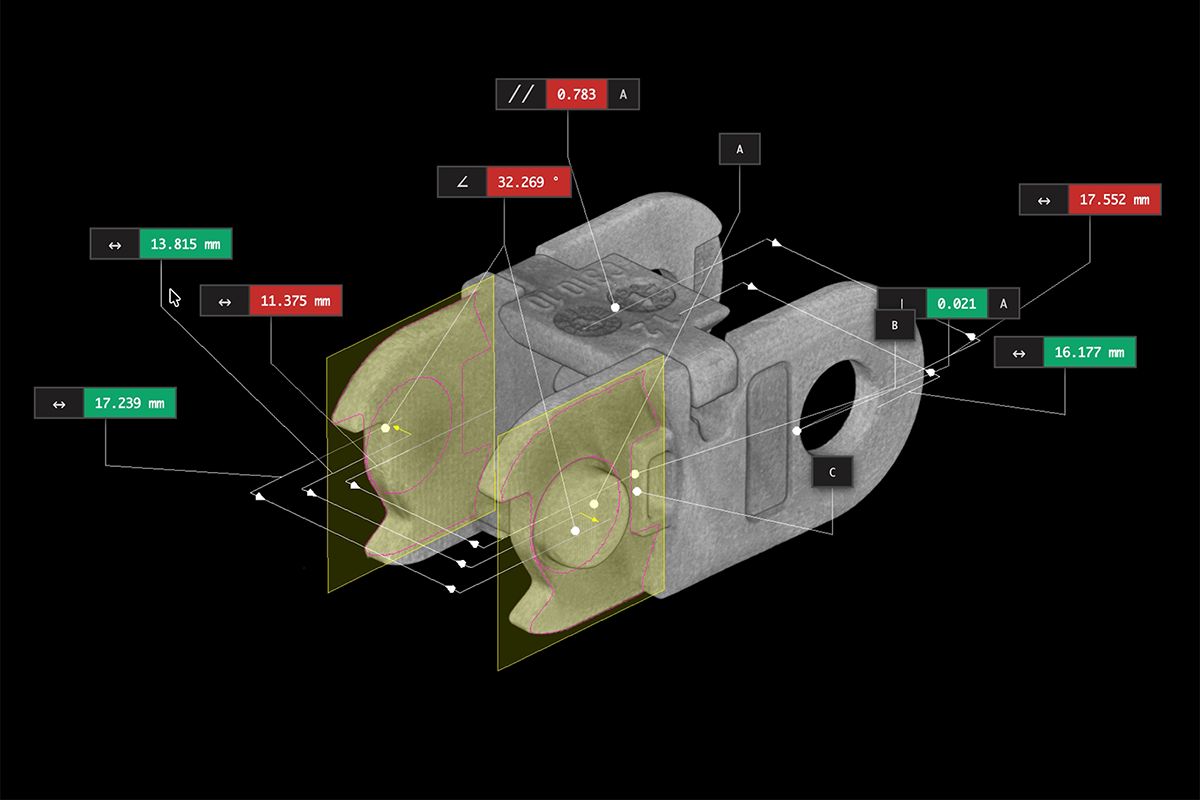
Despite obtaining FDA approval, approximately 4,500 medical devices are removed from shelves every year due to previously undetected defects. Legacy inspection methods are failing medical device makers and the people who rely on these life-saving devices.
Download this free guide, The Engineer's Guide to Medical Device Defects, to learn more about:
- The most common causes of medical device defects
- How industrial CT can detect defects before they cause recalls
- Why traditional inspection methods fail to detect common defects
- Where in the product development lifecycle CT fits in
- Why CT is more accessible and affordable than you think