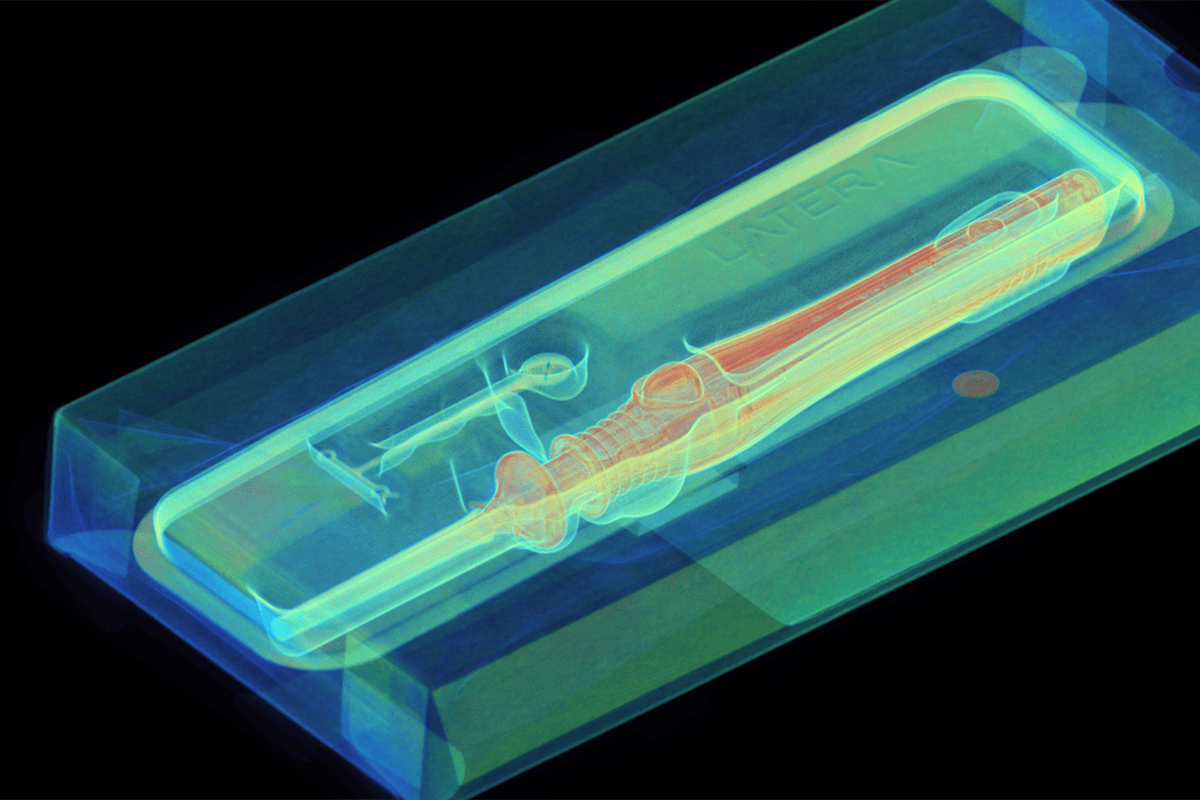
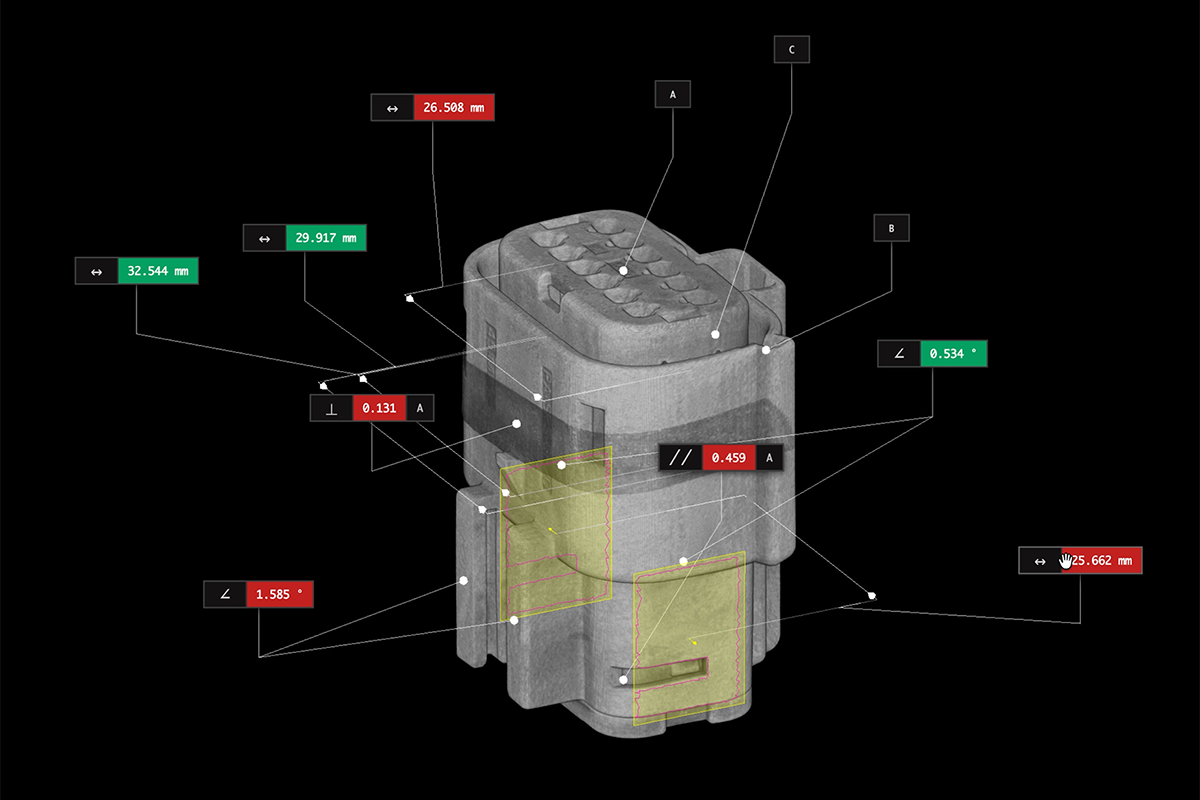
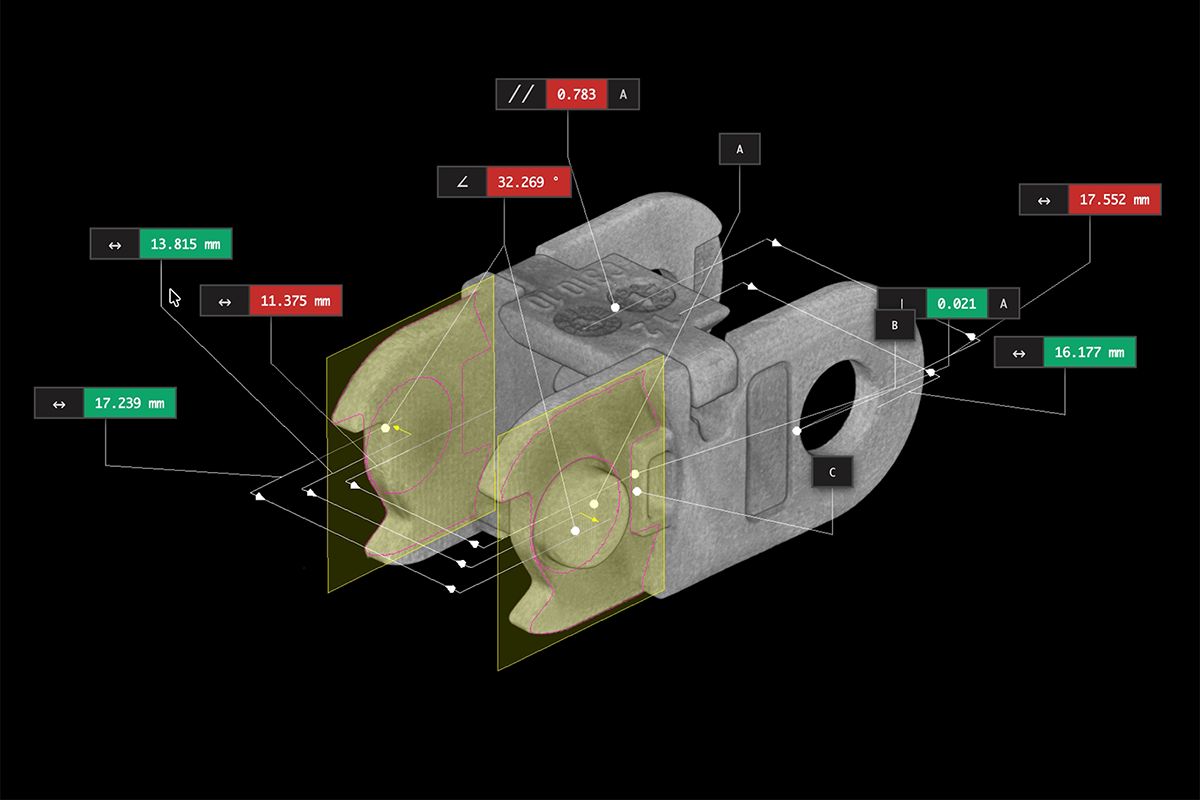
Medical device manufacturers face some of the strictest regulatory and quality standards in industry. This white paper outlines how industrial CT scanning helps engineers tackle challenges in design validation, process optimization, and compliance—without compromising speed or safety. It provides a focused look at six essential use cases across the product lifecycle.
Download this white paper and learn:
- How CT reveals hidden internal defects in complex assemblies
- Why high-resolution volumetric imaging supports compliance
- How engineers use CT data for FAI, supplier qualification, and tooling validation
- Ways CT supports continuous QA in production with non-destructive, high-speed scans
- How reverse engineering with STL files accelerates R&D and reduces development cycles
Upgrade your inspection process and see how CT scanning supports every stage of medical device development.